A QMS document structure is the hierarchical framework used to organize and align QMS documents to ensure a company’s products or services meet customer requirements. A well-structured QMS document system aligns with ISO 9001:2015 and IATF 16949:2016 quality management system standards and includes various levels of documentation.
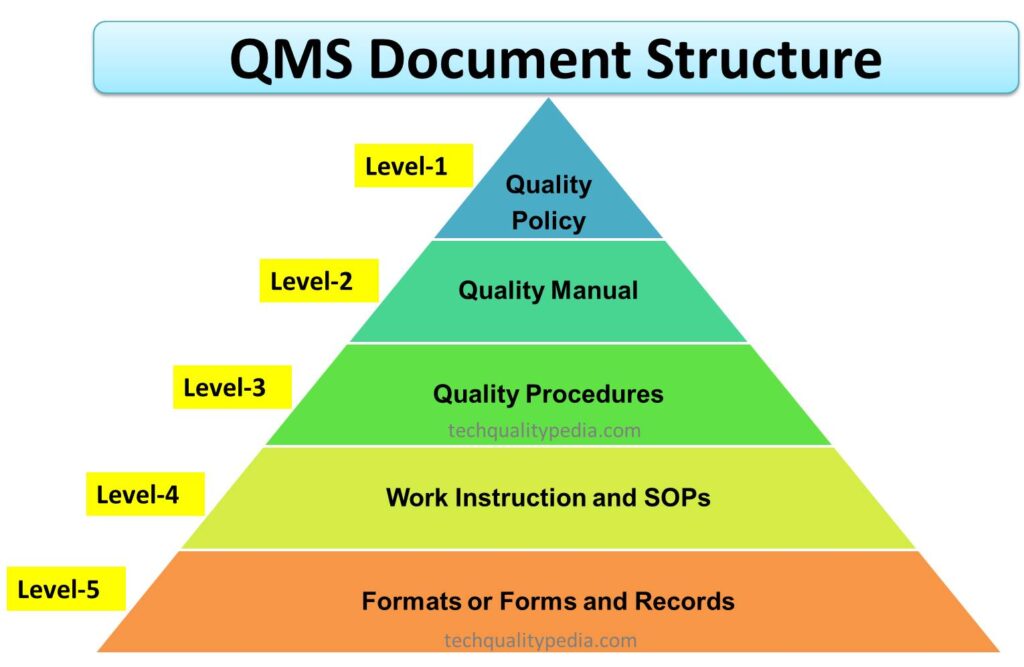
A QMS document structure, or QMS document hierarchy, or QMS Pyramid of the organization starts with the Quality Policy, which is generally established by the company’s top management (Managing Director). The second key document of the QMS document hierarchy is the Quality Manual, which includes quality policy, scope of the quality management system, quality objectives, linkages of key procedures/processes, and other quality management system requirements as per ISO 9001 and IATF 16949 QMS standard.
Table of Contents
QMS Document Structure
The QMS document structure or QMS document hierarchy of the quality management standard is briefly described below:
- Quality Policy (Top-Level)
- Quality Manual (Top-Level)
- Quality Procedures (Mid-Level)
- Work Instructions and SOPs (Lower-Level)
- Formats or Forms and Records (Evidence-Level)
Quality Policy
A Quality Policy of an organization is a formal statement from the top management that defines its commitment to quality. It provides a framework for establishing quality objectives and promoting continual improvements within a Quality Management System. The organization’s quality objectives and strategic direction must be aligned with the quality policy. The Quality Policy must be communicated and understood to all the interested parties, e.g. Customers, Suppliers, Employees, and contractors etc.
Quality Manual
- Purpose: Outlines the scope of the QMS, key procedures & processes, and the organization’s quality policy.
- Evidence: ISO 9001:2015 QMS standard clause 4.3 and 4.4 require the determination of the scope of the QMS and description of processes.
- Quality Manual covers the following documents:
- Scope of Quality Management System
- Organization’s Mission and Vision
- Quality Policy and Objectives or KPIs (Key performance indicators)
- Process Interaction Map or Diagram
- Linkage of Key Procedures and Documents
- Other QMS requirements as per ISO 9001 and IATF 16949 standard
Quality Procedures
- Purpose: Quality procedures are documented to describe how quality-related activities are performed.
- Evidence: ISO 9001:2015 Clause 7.5 provides documented information necessary for the effectiveness of the QMS.
- Examples of Quality Procedures:
- Document control and record procedure
- Training procedure
- Internal audit procedure
- Management Review System (MRM) procedure
- Customer satisfaction procedure
- Non-conforming products handling procedure
- Production planning and control proocedure
- ECN/PCN procedure
- New product development procedure
- Maintenance Procedure
- Purchasing Procedure
- Supplier selection, evaluation and development procedure
- Employee empowerment and motivation procedure
- Communication procedure
- Traceability procedure
Work Instructions and SOPs (Lower-Level)
- Purpose: WIs/SOPs provide detailed instructions for performing specific activities/tasks.
- Evidence: ISO 9001:2015 clause 8.5.1 supports the use of operating procedures to ensure conformity of production/service.
- Example of WI/SOPs:
- Measuring instrument calibration instructions
- Rework and repair instructions
- Operation standards or work standards or process quality check sheet (PQCS)
- Customer return handling instructions
- Material Handling and storge
- Bin management (Bin cleaning and repairing process)
Records and Formats (Evidence Level)
- Purpose: Records provide evidence of conformity and implementation of the system or procedures.
- Evidence: ISO 9001:2015 Clause 7.5.3 provides control of documented information as evidence of implementation.
- Examples of records and formats:
- Internal audit records
- Management review meeting records
- Equipment calibration record
- Inspection check sheet
- Daily machine check sheet
- Training attendance and effectiveness record
- Product inspection (Setup approvals, Incoming/Inprocess/Final/PDI Inspection) & test results
- Product and process validation record
- Preventive maintenance record
- Manufacturing process audit record
- Layout Inspection record
